
Human-Centered Design of Health Technologies
Human-centered design (HCD) focuses on problem-solving approaches based on the specific needs and wants of end-users and communities from the beginning to the end of the design process. We use this approach to bridge the gap between technology developers and communities that need health technologies.
Projects

Human Papillomavirus (HPV) Rapid Test for Cervical Cancer Screening
Environmental, social, and emotional factors create unequal barriers to cervical cancer screening. Cervical cancer screening is critical to the detection and prevention of cervical cancer. It is typically done at the doctor’s office via a pap test and/or HPV test. We are developing a HPV rapid test for cervical cancer screening to address screening gaps. The people who may use this device are sharing their insights, needs, and feedback so it can make screening more accessible for those who need it most.
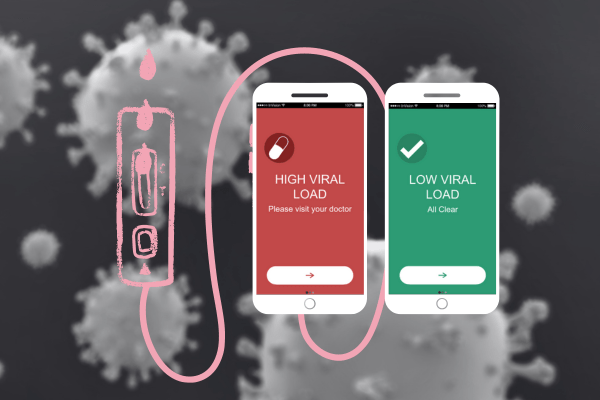
Smartphone-Based Self-Test for HIV Viral Load Monitoring
People living with HIV (PLHIV) who have an undetectable viral load cannot spread the virus to others. Viral load monitoring is the gold standard to measure treatment success. It is typically done at the doctor’s office. PLHIV and HIV providers have seen a prototype, developed by the Linnes Lab, of a HIV viral load self-test device that can connect to a smart-phone and deliver results within 30 minutes. They are helping us with future iterations of the device and best ways to implement this in the HIV care continuum.
Early Detection of HIV
Current HIV tests give results in under 20 minutes, but cannot detect HIV during the acute phase when it’s most infectious. The Linnes Lab developed a test that can detect HIV during this acute stage, but takes an hour to provide results. Is this a worthwhile trade-off? What are the testing needs and preferences for people who use drugs and other high-risk communities? We’re engaging with these communities and the organizations that serve them to find out.
Selected Publications
Rodriguez, N. M., Balian, L., Tolliver, C., Kataki, I., Jesus, J. R., & Linnes, J. C. (2023). Human-centered design of a smartphone-based self-test for HIV viral load monitoring. Journal of clinical and translational science, 7(1), e262. https://doi.org/10.1017/cts.2023.686
Rodriguez, N. M., Burleson, G., Linnes, J. C., & Sienko, K. H. (2023). Thinking Beyond the Device: An Overview of Human- and Equity-Centered Approaches for Health Technology Design. Annual review of biomedical engineering, 25, 257–280. https://doi.org/10.1146/annurev-bioeng-081922-024834
Rodriguez, N. M., Brennan, L. P., Claure, L., Balian, L. N., Champion, V. L., & Forman, M. R. (2023). Leveraging COVID-era innovation for cervical cancer screening: Clinician awareness and attitudes toward self-sampling and rapid testing for HPV detection. PLOS ONE, 18(3), e0282853. https://doi.org/10.1371/journal.pone.0282853
Rodriguez, N. M., Brennan, L. P., Claure, L., Balian, L. N., Kasting, M. L., Champion, V. L., & Forman, M. R. (2023). Clinician practices, knowledge, and attitudes regarding primary human papillomavirus testing for cervical cancer screening: A mixed-methods study in Indiana. Preventive Medicine Reports, 31, 102070. https://doi.org/10.1016/j.pmedr.2022.102070
Rodriguez, N.M. (2021). Participatory Innovation for HPV Screening to Accelerate the Elimination of Cervical Cancer. The Lancet Global Health, 9(5), e582-e583. https://doi.org/10.1016/S2214-109X(20)30522-2
Rodriguez, N. M., Wong, W. S., Liu, L., Dewar, R., & Klapperich, C. M. (2016). A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab on a chip, 16(4), 753–763. https://doi.org/10.1039/c5lc01392e
Collaborators
Funding Sources


